A Diagnostic Test Enabling Precision Oncology in Head and Neck Cancer
Predicts Therapeutic Response to ICI and Anti-EGFR Antibody Treatments
Overview
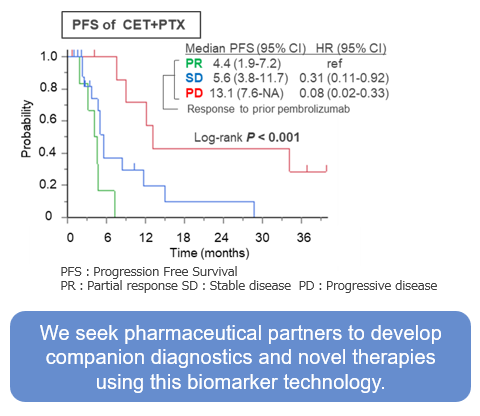
For patients with platinum-sensitive recurrent or metastatic head and neck squamous cell carcinoma (HNSCC), first-line therapy usually involves pembrolizumab (an immune checkpoint inhibitor; ICI) with chemotherapy (5-FU plus cisplatin/carboplatin), or pembrolizumab alone for PD-L1–positive cases defined by the combined positive score (CPS). As a second-line option, cetuximab (anti-EGFR antibody) plus paclitaxel (CET+PTX) is commonly used. Our study revealed a mutually exclusive correlation between responses to first-line pembrolizumab and second-line CET+PTX. Comprehensive gene expression analyses identified key biomarkers linked to this correlation. This diagnostic approach allows prediction of each therapy’s efficacy in individual patients by measuring these biomarkers.
Data
Application
These biomarkers enable identification of patients likely to respond to each therapy, supporting:
・prior selection of CET regimens,
・combination therapies with ICIs and anti-EGFR antibodies,
・combination therapies with ICIs and agents targeting the identified pathways.
IP Data
IP No. : JP2025-126227
Inventor : SAIJO Ken,KAWAKAMI Hisato
keyword : CDx, Cancer
