Immune checkpoint inhibitor
Drug discovery toward immune checkpoint LILRB4
Overview
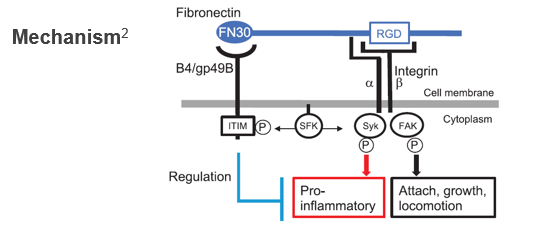
Conventional immune checkpoint (CP) inhibitors (e.g. PD-1 and CTLA-4 inhibitors) have revolutionized cancer immunotherapy, but are ineffective in approximately 40-80% of patients and have side effects such as autoimmune inflammation. The myeloid CP molecule LILRB4 (B4) has the unique property of being involved in immune evasion of cancer while also being involved in the exacerbation of autoimmune diseases, and is expected to be a new drug target, but its ligand was unknown. The present invention identifies fibronectin (FN) as the only physiological ligand for B4 and finds that immune control is possible by inhibiting the binding of B4 to FN, and relates to a novel CP inhibitor based on this finding.
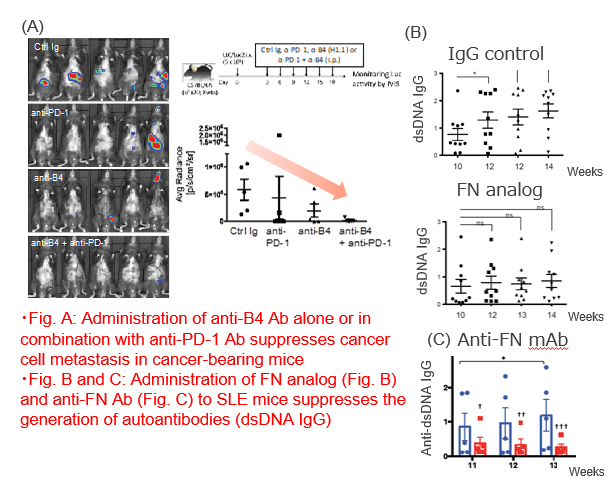
Following patterns can be considered for immunoregulation by blocking the binding between B4 and its ligand FN :
(1) FN analog (competitively binds to B4-FN)
(2) Anti-B4 antibody (acts on B4 and inhibits B4-FN binding)
*Company X has Phase 1b data for its in-house anti-B4 antibody in cancer immunotherapy. Data disclosure may be possible through negotiation (terms to be determined).
(3) Anti-FN antibody (acts on FN and inhibits B4-FN binding)
Further, B4 as a biomarker for lung cancer patients’ prognosis prediction was verified by our original B4 monoclonal antibody that inhibits B4-FN binding (data not shown).
Features・Outstandings
Product Application
・Drugs for treating autoimmune, cancer, inflammatory or allergic diseases associated with B4
・Diagnostic agent that predicts the effectiveness of cancer immunotherapy
Related Works
[1] International Immunology, 2021; 33(8), pp. 447–458.
[2] International Immunology, 2022; 34(8), pp. 435–444.
IP Data
IP No. : JPB7740705, WO2021/029318
Inventor : TAKAI Toshiyuki, SU Mei Tzu, et al.
keyword : checkpoint inhibitor, antibody, oncology, LILRB4, ILT3
